i4pd began working with Optos in 2010 on a project that aimed to fundamentally change the image of the company’s product line. A year and a half later and the ‘Daytona’ retinal imaging device could be found in many high street optometrists. Since then we have worked together on a range highly complex projects to create a family of products with a consistent look and feel.
Optos, now a Nikon company, always had an ambition to establish themselves as “The Retina Company”. To make this a reality, Optos had amassed a wealth of optoelectronics, electromechanical and software experience in-house to design and refine their unique ultra-widefield imaging platform. To complement this central product development function, Optos were keen to work collaboratively with an industrial design partner who could support an improved patient interface and cover design to create something special, the likes of which had never been seen in the optometry & ophthalmology markets.
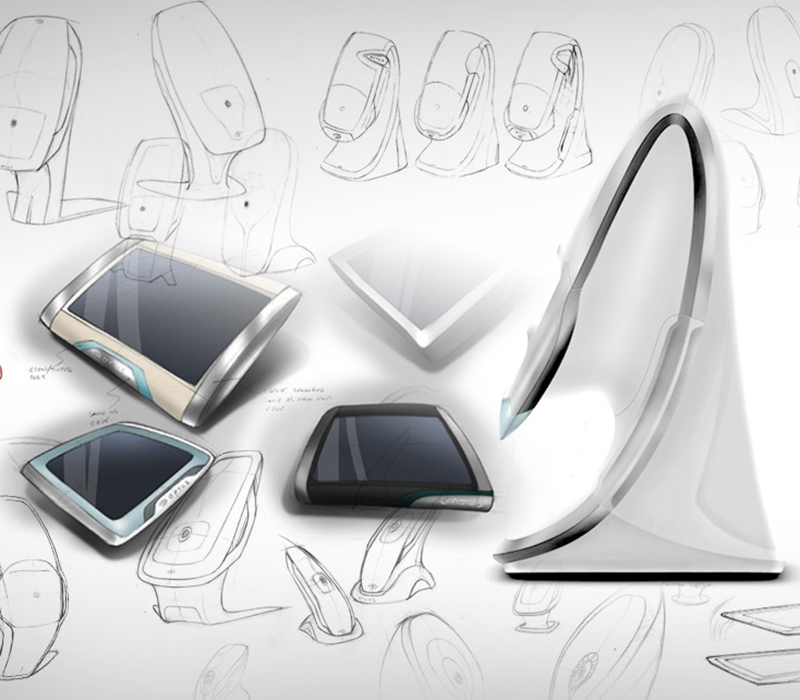
Each of the 4 retinal scanners i4pd worked on with the Optos engineering team contained a different array of imaging modules. Each were designed to deliver the optometrist or clinician with different image modalities. With these different types of images, a patient could be seated in front of the device for anywhere between 0.5 second to 90 seconds. Patients may also be imaged whilst in a wheelchair and/or injected with a fluorescent dye to aid with visualising the blood vessels. The company had not previously designed products with large injection moulded enclosures but, due to the cost targets and aesthetic aspirations, this was a necessary transition away from their previous manufacturing processes.
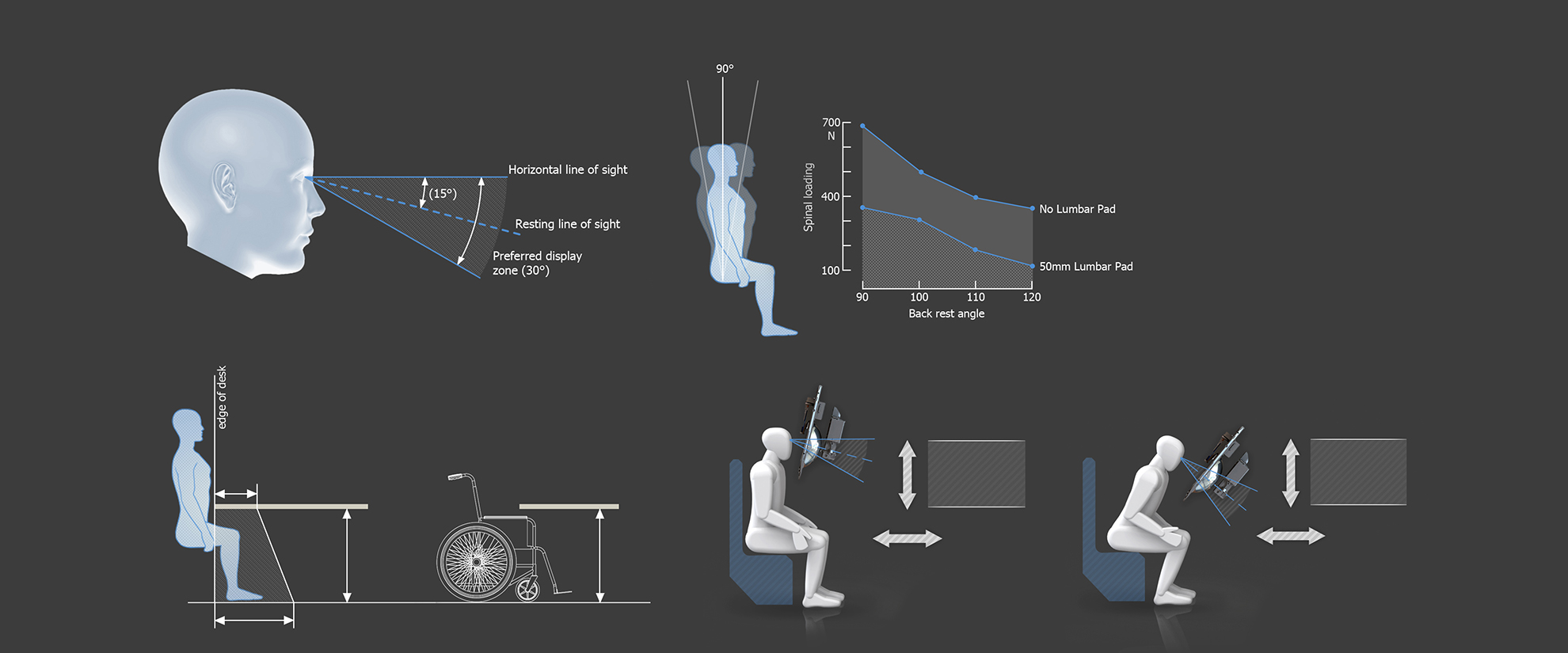


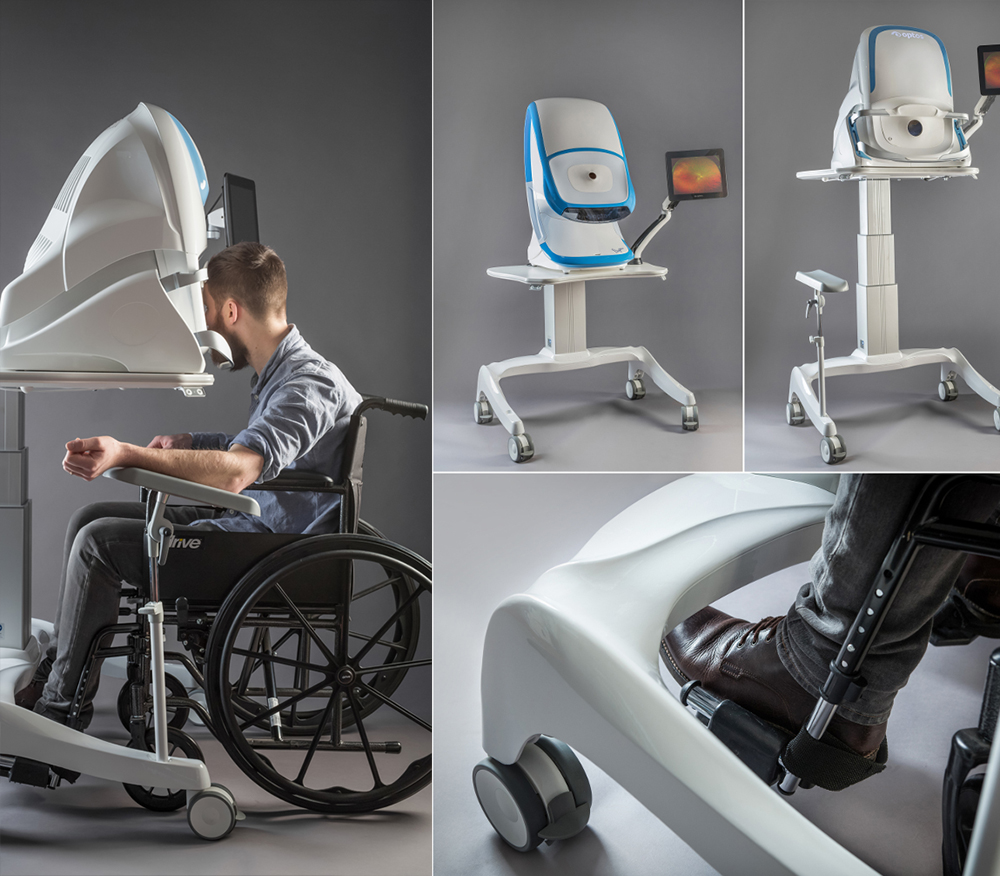
The devices presented themselves as particularly user-centred products which meant we prioritised our effort on the system’s ergonomic design. We studied anthropometric data and carried out extensive user tests to ensure we optimised patient comfort. As instinctive designers we focused on the form factor of the device as well as considering the design’s manufacturing and production implications. In this case, a soft patient interface that required cleaning after each use and large, complex injection mouldings for the device covers.
We worked collaboratively with Optos' engineering team through the pre-production phase, responding as needed with the detailing of design concepts and discussions with specialist moulders.


In comparison to Daytona, which was marketed at high street optometrists, the other devices (Monaco, Silverstone and California) were developed for global use in ophthalmology offices and hospital environments. We worked closely with clinicians to get an understanding of the user work flow, to enable us to experience the ergonomics from the point of view of patients and hospital staff. One of the main design challenges was to optimise the design to suit a wide range of population including wheelchair users. Based on our observations and experience with conceptual test rigs we produced a series of refined ergonomic test platforms that informed the development process.

The complicated X-Y-Z range of motion in these devices really set themselves apart from the earlier Daytona. We were heavily involved in designing the electro-mechanical operation of the device which had its own challenges of space and weight constraints - all of which had an impact on the overall design.
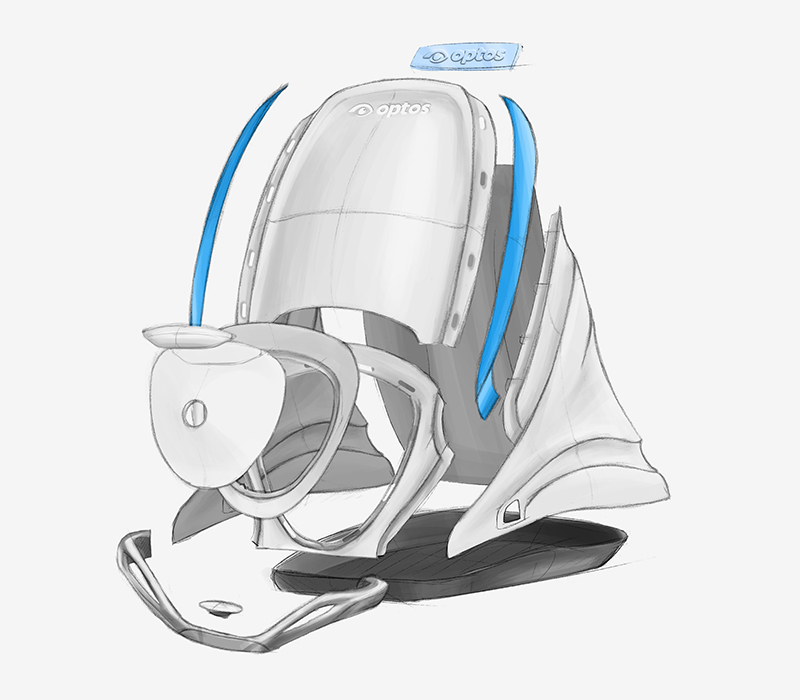
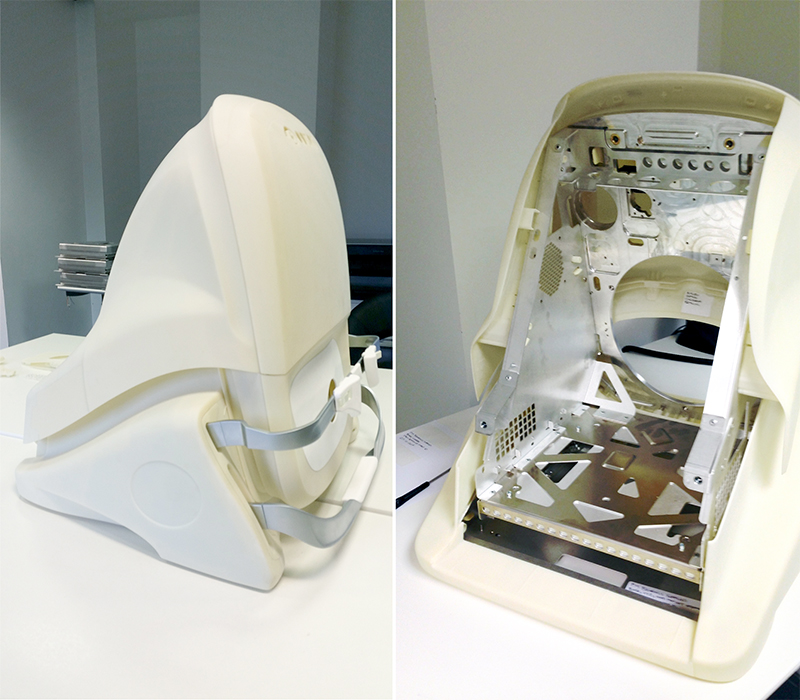
Prototyping played a key role to the success of each project. It involved the verification of user design during the concept generation phase but also of engineered parts for the Alpha and Beta phases. Not only were the prototypes integral to informing i4pd’s design team in the development of the device, they were also important for Optos’ clinical testing and marketing requirements. Being a medical device that would be used in a hospital environment, certain standards and regulations had to be adhered to in the design. Drawing from our UK and international prototype vendor base we produced vacuum formed and CNC parts using clinical grade materials with non-flammable specifications.
As with most of our projects, i4pd’s work extends beyond the design and engineering phases. Having gone through the process of developing the product architecture through to detailed engineering design of the final plastics, we also provided support in tooling and manufacturing for our client where we liaised weekly with manufacturers in the UK and overseas. We helped to verify tool designs and ensured that all the plastics produced were to design intent and to the standards of finish expected of a high value product. Critical documentation and reporting which monitored manufacturing progress was also part of i4pd's remit, strengthened with our ISO 13485 quality management system.

We like to find new, better and efficient ways of doing things. Contact us to discover how i4 Product Design can solve your current design challenge and take your product to the next level.
Copyright © 2024 i4 Product Design Ltd. All rights reserved. | Privacy Policy